Blood Collection Guideline
I. Purpose
The purpose of this guideline is to provide recommended blood sampling volumes and guidance on a variety of acceptable blood collection techniques in rodents and bats.
II. Scope
This guideline applies to all personnel collecting blood samples from laboratory rodents and bats.
III. Guidance
- General Information
- Factors to consider when selecting the appropriate blood collection technique for
research purposes include, but are not limited to:
- The species to be bled
- The size and age of the animal to be bled and the estimated total blood volume
- The type of the sample required (e.g., serum, whole blood cells, )
- The quality of the sample required (sterility, tissue fluid contamination, )
- The quantity of blood required (considering extraneous blood loss due to a selected method)
- The frequency of sampling
- The health status of the animal being bled
- The training and experience of the phlebotomist
- The size and type of capillary tube is appropriate
- The effect of the site, restraint, or anesthesia on the blood parameter measured.
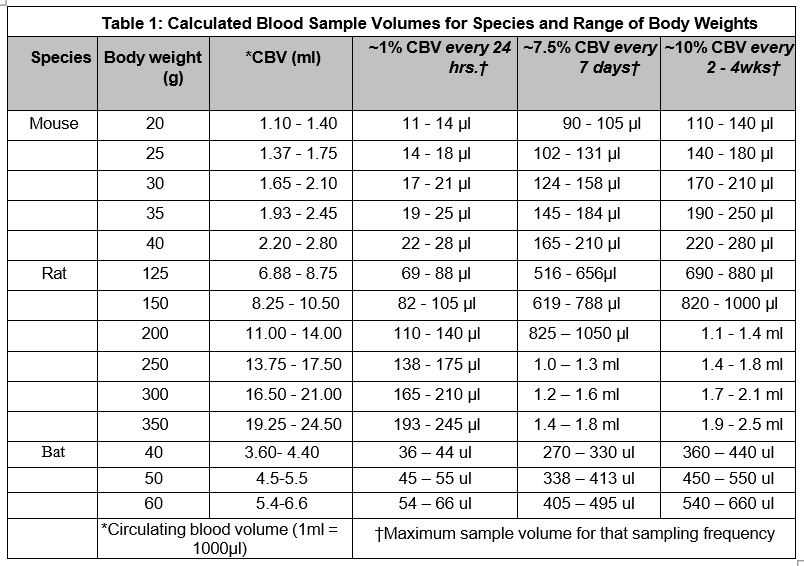
- The acceptable quantity and frequency of blood sampling is dependent on the circulating
blood volume of the animal and the red blood cell (RBC) turnover rate. The approximate
circulating blood volume of adult animals varies with species and body weight. For
purposes of calculating the maximum blood volume that may be sampled, the following
reference mean total blood volume (TBV) values may be used:
- Mouse 72 ml/kg
- Rat 64 ml/kg
- Hamster 78 ml/kg
- Guinea pig 75 ml/kg
- Bats 95 ml/kg
- Approximately 10% of the total blood volume can be safely removed every 2 to 4 weeks, 7.5% every 7 days, and 1% every 24 hours.
- The guidance provided below is for healthy adult animals. Animals that are young, aged, stressed, have cardiac or respiratory disease, or are otherwise compromised may not tolerate recommended amounts of blood removal.
- If the experimental design requires blood volumes and/or frequency of collection that fall outside the recommendations within this guideline, consultation with the AV and justification in the IACUC protocol is required.
- Factors to consider when selecting the appropriate blood collection technique for
research purposes include, but are not limited to:
B. Collection site requirement and advantages / disadvantages:
| Collection Sites | Species | ADVANTAGES | DISADVANTAGES |
| Submandibular Sampling | Rodent |
|
|
| Tail Nick or Tail Vein Sampling | Rodent |
|
|
| Sublingual Vein | Rodent |
|
|
| Saphenous Sampling (medial or lateral approach) | Rodent |
|
|
| Cardiac Puncture |
Bat Rodent |
|
|
| Retro-orbital Sinus | Rodent |
|
|
| Wing Vein Sampling | Bat |
|
|
C. References:
-
- Diehl KH, Hull R, Morton D et al: A Good Practice Guide to the Administration of Substances and Removal of Blood, Including Routes and Volumes. J Appl Toxicology 21: 15-23, 2001.
- Montani, DJ, Cooper, DM: Management of Animal Welfare Issues following Retroorbital Blood Collection in Rats. Techtalk Vol.14/No.3, 2009.
- Hawk TR, Leary SL and TH Harris (eds).: Formulary for Laboratory Animals, 3rd Blackwell Publishing, Ames, Iowa, 2005.
- McGill MW and AN Biological Effects of Blood Loss: Implications for Sampling Volume and Techniques. ILAR News 31(4): 5-18, 1989.
- Removal of Blood from Laboratory Mammals and Birds: First Report of the BVA/FRAME/RSPCA/UFAW Joint Working Group on Refinement. Laboratory Animals 27: 1-22, 1993.
- The UFAW Handbook on the Care and Management of Laboratory Animals, Volume 1. CRC Press, New York, 2003. “Blood Sampling: pp 379-386.
- Raabe BM, Artwohl JE, et al: Effects of Weekly Blood Collection in C57BL/6 Mice. JAALAS 50(5):680-685, 2011.
- Hoff, Janet: Methods of Blood Collection in the Lab Animal 29(10): 47-53, 2000
- Scipioni, RL; Diters, RW; et al: Clinical and Clinicopathological Assessment of Serial Phlebotomy in the Sprague Dawley Rat. 47(3):293-299. 1997
- Hui, Yu-hua, Huang NH, et al: Pharmacokinetic comparison of tail-bleeding with cannula- or retro-orbital bleeding techniques in rats using six marketed drugs, Journal of Pharmacological and Toxicological Methods 56:256-264,
- Shirasaki, Y; Ito Y, et al: Validation Studies on Blood Collection from the Jugular Vein of Conscious Mice: JAALAS, 51(3): 345-351, 2012.
- NIH Rodent Blood Collection Guidelines: http://oacu.od.nih.gov/ARAC/documents/rodent pdf
- Joslin Blood Collection Techniques in Exotic Small Mammals. J. of Exotic Pet Medicine. 18:117-139, 2009.
- Gold WT, Gollobin P. and Rodriquez LL. A rapid, simple, and humane method for submandibular bleeding of mice using a Lab Animal. 34:39-43, 2005.
- Beeton C, Garcia A, and Chandy Drawing Blood from Rats through the Saphenous Vein and by cardiac Puncture. J Vis Exp. 7:266, 2007
- Parasuraman S, Raveendran R, Kesavan R. Blood sample collection in small laboratory J Pharmacol Pharmacother. 2010 Jul;1(2):87-
- doi: 10.4103/0976-500X.72350. Erratum in: J Pharmacol Pharmacother. 2017 Jul-Sep;8(3):153. PMID: 21350616; PMCID: PMC3043327.
- Regan RD, Fenyk-Melody JE, Tran SM, Chen G, Stocking KL. 2016: Comparison of Submental Blood Collection with the Retroorbital and Submandibular Methods in Mice (Mus musculus). J Am Assoc Lab Animal 2016; 55(5):570-6.
- Heard DJ. Zoo Animal And Wildlife Immobilization And Anesthesia. 2007. pp. 359–365.
IACUC Approval Date: 2/19/2020
Review Date: 7/16/2025
Issue Date: 7/23/2025