Diseases of Cool Season Legumes
(Pulse Crops: Dry Pea, Lentil, and Chickpea)
By Uta McKelvy, Assistant Professor Extension Plant Pathology; Erin Gunnink Troth, Research Associate; and Mary Burrows, Associate Dean for Research and Director of Virginia Agricultural Experiment Station
Plant Sciences and Plant Pathology Department, Montana State University, Bozeman, MT College of Agriculture and Life Sciences, Virginia Tech, Blacksburg, VA
Table of Contents:
Disease Problems at Establishment
Disease Problems During the Growing Season
Glossary
For Further Information
As pulse crops are more frequently included in crop rotations, we see increased disease problems. Correct diagnosis is the first step toward successful disease management. In this publication, we discuss the symptoms of various diseases of pulse crops commonly occurring in Montana, including some diseases of concern for the future, and their management.
DISEASE PROBLEMS AT ESTABLISHMENT
Establishing a good plant stand is important to reduce competition from weeds and protect plant health throughout the growing season. Using healthy seeds with high germination, an appropriate variety, crop rotation, and seed treatments can go a long way toward protecting a crop.
Damping off
Poor plant emergence is often the first sign of damping off. Damping off can occur before emergence or in the young seedling (post-emergence damping off). Weak stand establishment, yellow seedlings with no secondary roots or a brown or black tap root, and seedling death soon after emergence are characteristic symptoms (Figure 1).
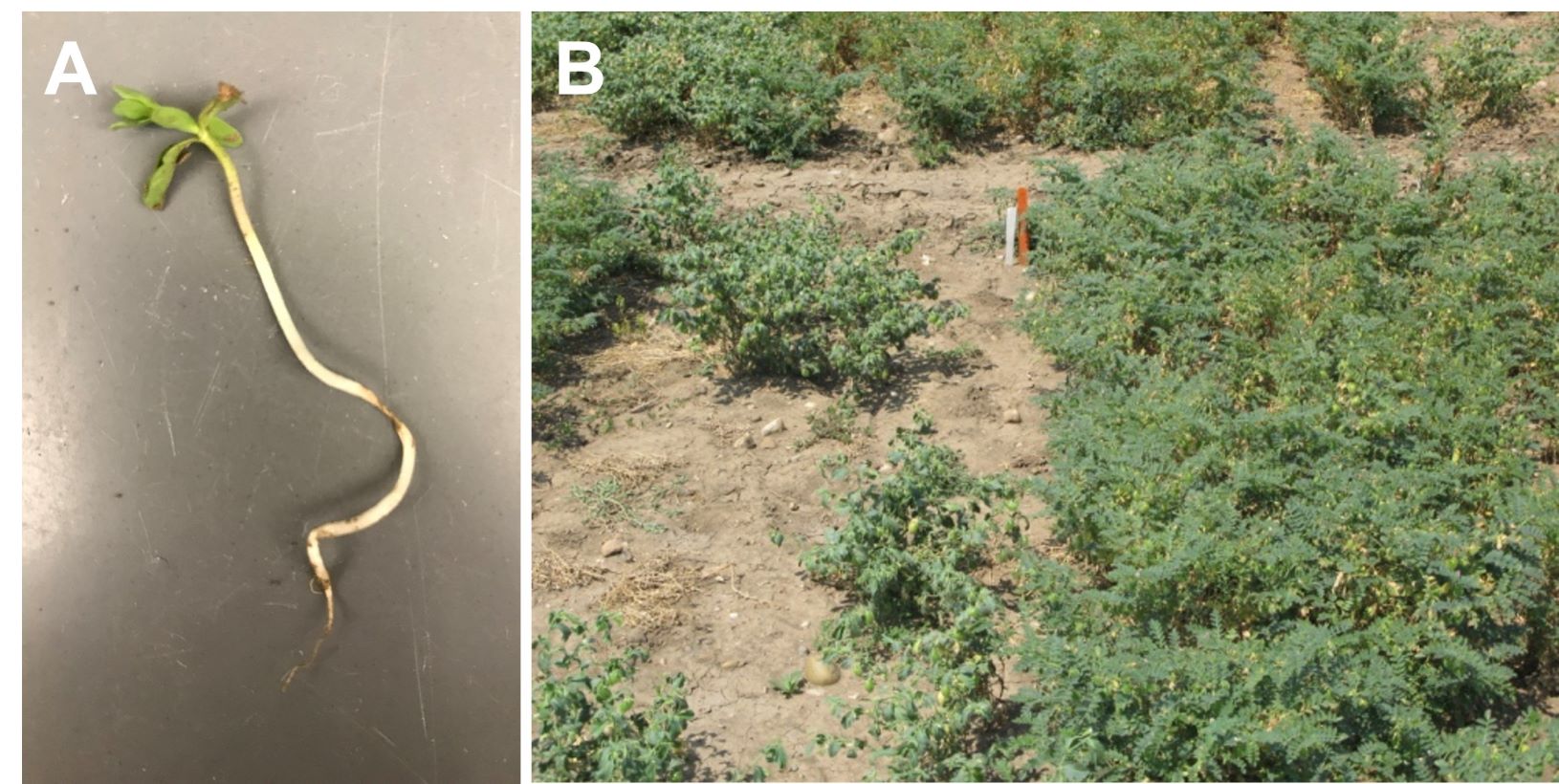
Figure 1. (A): Damping off is the result of a fungal infection girdling the developing seedling. (B) Note the significant loss of stand due to damping off. Kabuli chickpeas (left) are more susceptible to damping off than desi chickpeas (right).
Damping off is caused by several pathogens including Pythium spp., Fusarium spp., and Rhizoctonia spp. These pathogens are soilborne. Disease development is favored by cool, wet soils at spring planting. No-till management can increase the risk of damping off. Kabuli-type chickpeas are highly susceptible to Pythium seed and seedling rot. Light-colored lentil seeds are much more susceptible to Pythium than dark-colored seeds, which contain tannins that inhibit the pathogen.
Damping off can be prevented by planting vigorous seeds to ensure rapid germination. Delay seeding until soil temperatures have increased above 50°F. Fungicide seed treatments are strongly recommended for all pulse crops. The seed treatment should contain metalaxyl or mefenoxam for control of Pythium in addition to another fungicide for control of Fusarium, Rhizoctonia, etc. The selection of seed treatment will depend on what is available at retailers. Consult the ‘Montana State University Seed Treatment Fungicide Table for Pulse Crops’, the 'North Dakota State University Field Crop Plant Disease Management Guide' or the ‘High Plains Integrated Pest Management (IPM) Guide’ to make the best decision for the crop production system (see links under “Helpful websites and contacts” at the end of this publication).
Root and Crown Rots

Figure 2: Above-ground yellowing caused by below-ground root rot.
Environmental conditions leading to plant stress, or facilitating pathogen growth, will result in root rot after crop emergence. Above-ground symptoms include stunted, yellow plants that may be mistaken for nitrogen deficiency (Figure 2). The root systems of diseased plants will be much thinner than those of healthy plants or there may be no secondary roots at all. Roots will be discolored, and the color and pattern of discoloration depend on the causal pathogen. There are four main types of root rot: Pythium root rot, Rhizoctonia root rot (bare patch), Fusarium root rot, and Aphanomyces root rot. Differentiation between the root rots based on symptoms is not always clear-cut because more than one pathogen often contributes to root rot.
Root rot-causing pathogens are soilborne. Sanitation strategies that prevent the spread of infested soil between fields on field machinery and boots should be considered. Many of the root rot pathogens have a broad host range, which limits the efficacy of crop rotation as a management tool. However, long crop rotations with a minimum of three- to four-year breaks between pulse crops are strongly recommended to reduce the build-up of high pathogen populations in the soil. Once root rot pathogens become established, a minimum four-year break may not be sufficient to reduce disease. Cultural practices that promote soil health and fertility, adequate soil moisture, and root growth can reduce disease severity. Root rot-resistant varieties are not currently available. Resistance to root rots is quantitative. Pulse crop breeding programs are working to incorporate resistance traits into new varieties, but the incorporation of resistance into commercially available varieties can be a slow process.

Pythium root rot is caused by different species of the water mold (Oomycete) Pythium. The disease is characterized by poor stand establishment and can be difficult to diagnose. In general, infected plants have few lateral roots, existing roots have brown lesions, and the outer cortex may slough off the inner core of the root (Figure 3). This disease is especially favored by cool, wet soils. Pythium is very common in the soil, and for this reason, it is recommended that all seed treatments contain metalaxyl, mefenoxam, or ethaboxam where metalaxyl-resistant populations have been detected. However, most seed treatments provide protection for only two to three weeks after planting. If disease-conducive conditions persist longer than seed treatments maintain efficacy, plants may still become infected.

Figure 4: Root systems infected with Rhizoctonia root rot are much reduced and have reddish to dark brown lesions.
Rhizoctonia root rot is caused by the fungus Rhizoctonia solani. It is first indicated by poor or declining stands. Root development is poor, and roots will develop reddish to dark brown lesions that may pinch off the root beyond (root pruning; Figure 4). Rhizoctonia root rot is very common where volunteer grain and weeds are sprayed with herbicide, particularly glyphosate, within days of planting. The fungus grows quickly on the dying plants and reaches a high population which then may attack new seedlings. This is called the ‘green bridge.’ The green bridge can be prevented by delaying seeding two to three weeks after herbicide application to allow the plant tissue to decompose. Other management practices include the use of a fungicide seed treatment for good plant establishment, planting into well-drained soils, and crop rotation to non-host crops such as corn and small grains.

Aphanomyces root rot is caused by Aphanomyces euteiches. This water mold (Oomycete) can affect seedlings and older plants. Infected roots show a characteristic honey- or caramel-brown discoloration (Figure 5). At advanced stages of infections, the root system disintegrates, and plants become chlorotic, wilted, and may die prematurely. Warm soil temperatures above 60°F, high soil moisture, and poor drainage favor disease development, as do heavy clay soil and/or compacted soil. The pathogen produces thick-walled oospores that can survive in the soil for eight to 10 years or more. Spring-planted peas and lentils are most susceptible to the disease, while chickpeas are moderately resistant. Genetic resistance to Aphanomyces is quantitative and to date, there are no commercial lentil or pea varieties with high levels of resistance available. Metalaxyl and mefenoxam have no efficacy on the pathogen. Ethaboxam is currently the only seed treatment fungicide labeled for control of this disease, however, its efficacy is limited because the pathogen can cause disease beyond the timeframe when the chemistry provides protection. It is important to employ agronomic management strategies, such as long rotations with non-host crops to suppress inoculum buildup in the soil and avoid planting susceptible crops in heavily infested fields.
Fusarium root rot is caused by several different Fusarium species. Below-ground symptoms include a lack of secondary roots and brown to black lesions that move up and downward from the seed attachment site. Fusarium grows in the soil and causes disease at temperatures above 64°F. Root rot severity is promoted by environmental conditions inducing stress in the crop, such as fluctuating water conditions, drought, soil compaction, poor soil fertility, herbicide injury, soil pH below 5.1 or above 7.5, and poor seed vigor. Management recommendations include fungicide seed treatments and good weed control. No cultivars with high levels of resistance are available, but partially-resistant cultivars are in development. The severity of the infection often depends on the crop history and Fusarium species present in the soil. For example,F. graminearum, which causes root and crown rot in wheat, does not infect pea very well. F. redolens, which is decimating to peas, can be found on wheat at low levels causing root rot, but is not a major pathogen of wheat. F. avenaceum and F. solani are the most damaging species on pea roots in North America, and recent research has found that F. avenaceum is also a dominant species causing Fusarium head blight in smallgrains in Montana. Crop rotation can therefore be a more or less effective strategy in managing Fusarium root rot, depending on the dominant Fusarium species in a given field.
DISEASE PROBLEMS DURING THE GROWING SEASON
Vascular wilts
Fusarium wilt is caused by Fusarium oxysporum. Subspecies are specific to the host crop (i.e., F. oxysporum f. sp. pisi on pea, F. oxysporum f. sp. lentis on lentil, and F. oxysporum f. sp. ciceris on chickpea). There can be several different races of the pathogen within each subspecies, which makes breeding for resistance challenging. However, there is resistance in many pea varieties to the Fusarium wilt pathogen race 1 and race 2.
Symptoms include downward curling of stipules and leaflet margins, thick and brittle stems at the soil line, discoloration of the vascular tissue (orange-brown or reddish to black), and discoloration and rotting of secondary roots. Asymmetric leaflet size and wilting leaves while the stem remains green are additional symptoms. If the plant is infected when it is older, a black canker may develop on the stem close to where the pod is attached and enlarge upwards and downwards from that point. Diseased plants die more rapidly under dry soil conditions and occur in circular to oval patches. Plants may be more scattered if the level of inoculum is low or unevenly spread. Relatively warm soil temperatures (74°F to 82°F) are optimal for disease development.
F. oxysporum can infect the plant during any stage of growth. The pathogen is very long-lived in the soil and can increase in a field each time a susceptible crop is re-planted. For that reason, it is important that wilt-affected fields are entered last, and equipment is cleaned after each field operation. This is especially important for operations where there is soil contact, such as at planting. The pathogen can also be transported on plant materials such as stubble and in wind and water. It may also be introduced on seed. The only economical control is to plant resistant varieties. Early planting allows the crop to grow when soil temperatures are below those optimal for disease development. Seed treatments to encourage establishment and plant vigor are recommended. A minimum of four years between host crops is advised to curtail the progress of this and other soilborne root diseases.
Verticillium wilt is caused by Verticillium dahliae and V. albo-atrum. It is reported as an economic disease only on chickpea, although V. dahliae can infect lentil and pea. The symptoms are very similar to those of Fusarium wilt. The foliage turns yellow before wilting, and the xylem becomes light brown in color when the stem is split. Verticillium wilt is not considered a major disease in the Northern Great Plains but can be serious where it occurs. Crop rotation to non-host crops, such as small grains, is the most effective method of control. Use resistant chickpea varieties where available.
Foliar diseases
Foliar diseases can be some of the most devastating diseases of pulse crops and are often noticed too late to implement management. It is very important to scout crops regularly, particularly when humidity is high, and following rain and hail events. Early action against foliar fungi such as Ascochyta blight can be very beneficial. However, growers must be careful to discriminate between fungal diseases and bacterial diseases, for which there are no chemical control options.
Bacterial blight is very commonly seen in pea and chickpea and often occurs following events that inflict mechanical injury to the crop. Hail, rain associated with high winds, sandstorms, and frost create wounds on plants where the pathogen attacks. Bacterial blight is caused by Pseudomonas syringae pv. pisi and Pseudomonas syringae pv. syringae in pea, and Xanthomonas campestris pv. cassiae in chickpea. It can be easily confused with the fungal disease Ascochyta blight, but management is very different. Bacterial blight causes water-soaked lesions at first (when held up to the light, one can see that the tissue is translucent) which could be confused with physical damage from hail early in disease development (Figure 6). Lesions are limited by leaf veins, are angular, may be shiny from the dried bacterial ooze, and are associated with patterns caused by hail such as damage on the side of the plant exposed to prevailing winds. In contrast, lesions associated with Ascochyta blight are not limited by leaf veins, are circular, and may have pycnidia (small dark dots) associated with them. As the tissue dries, lesions caused by bacterial blight turn dry and brown, which can cause tearing of those lesions on the leaf edges. Disease symptoms can also occur on stems, petioles, and pods. If weather conditions remain humid after infection, the disease can progress very rapidly and defoliate plants. One may see cloudy droplets of bacteria along the edges of lesions which become crystalline and flake off as the leaf dries (Figure 6). If weather conditions become warm and dry after infection, disease progression may stop. It is common to see symptoms only on lower leaves, in cases where the crop has grown out of the disease. Yield losses are proportional to the amount of canopy lost.

Figure 6. (A) Symptoms of bacterial blight on pea leaf. Angular lesions turn brown when the leaf tissue dies. Bacteria ooze from the lesions under high moisture conditions and dry to white flakes on the leaf surface. (B) Water-soaked lesions and bacterial ooze where hailstones hit a petiole.
The pathogens are seedborne, and contaminated seed is an important source of inoculum for field epidemics. Using clean seed is therefore an important means of managing bacterial blight. Residue from an infected pea crop can also be an important source of inoculum, but pathogen survival can be reduced by crop rotation or burying residue. Sanitation of equipment can further reduce disease spread between fields and subsequent crops.
Alternaria blight, caused by Alternaria alternata, occurs sporadically on chickpea and lentil and is considered of minor importance. The disease can be easily confused with Ascochyta blight. The pathogen infects over 400 plant species. Temperatures of 75°F to 82°F and high relative humidity (>85%) favor disease development. The pathogen is seedborne and infested seed serves as the primary source of inoculum. Seed treatments can protect seedlings for up to 40 days after planting. Symptoms include pale brown lesions on leaf margins and tips, particularly on older leaves. Affected leaflets drop off the plant. There may also be dark brown, elongated lesions on stems, petioles, flowers, and pods.
Anthracnose of lentil is caused by the fungus Colletotrichum truncatum. It was first discovered in Canada and has since been found in North Dakota and Montana. Where it occurs, it can be a very serious disease. Symptoms include tan lesions that begin on the lower leaves at around 8 to 12 nodes of growth or first flower (Figure 7). Infected leaflets wilt and drop to the ground prematurely, which is characteristic of Anthracnose and may be the first sign of a serious disease problem. Lesions spread to stems and pods and can defoliate plants. Deep tan lesions, often with dark borders, can indent the stem (Figure 8A). Black fruiting structures and microsclerotia often become visible in the lesions. Defoliation and girdling can cause plants to wilt and lodge. Seeds from infected plants may be discolored and shriveled, resulting in significant dockage. The pathogen prefers temperatures of 68°F to 75°F and 24 hours of leaf wetness. The disease is polycyclic and can rapidly spread within a field under favorable conditions.

Figure 7. (A) Tan lesions on leaflets are early symptoms of Anthracnose. (B) As symptoms progress, they cause premature defoliation and plant death.

Figure 8 (A): Black fruiting structures and microsclerotia often become visible in the tan lesions caused by Anthracnose on lentil. (B) Anthracnose is easily confused with Ascochyta blight.
Microsclerotia of the fungus survive on infested debris and can remain viable for up to three years. Inoculum often comes from debris or seed, and windborne debris can carry the pathogen for many miles. Tillage of infested fields should be avoided because the pathogen breaks down quicker on the soil surface when exposed to extreme temperatures and repeated wetting and drying in regions with long, cold winters. This disease is best prevented by avoiding introducing the pathogen into an area via contaminated seed. There are foliar fungicides available with good efficacy against Anthracnose (see “Fungicide Use Information” at the end of this publication). They should be applied just before canopy closure or at the first signs of disease (premature leaf drop). Good coverage of the lower stems is important as infection of the main stem is most likely to cause plant death and severe yield loss. Multiple applications for disease control may be necessary throughout the season.
Ascochyta blight is a yield-limiting disease for chickpea production and may require multiple fungicide sprays for management. Peas and lentils are less susceptible to Ascochyta blight, but the disease can still require careful management depending on the timing and severity of infection, and the prevailing weather conditions. The species of Didymella (formerly Ascochyta) causing Ascochyta blight are host-specific: Didymella rabiei infects chickpea but will not infect pea or lentil; Didymella lentis infects lentil; three different species infect pea: Didymella pisi, Peyronellaea (formerly Mycosphaerella) pinodes, and Peyronellaea (formerly Ascochyta) pinodella.
Lesions associated with Ascochyta blight on chickpea are very distinct (Figure 9A and B). They are circular or oblong in shape and may begin as small, light-colored specks on the leaf which expand into target-shaped lesions. Each ‘wave’ of the lesion is surrounded by a brown-to-black halo, creating a target pattern over time. Lesions can also occur on stems, petioles, and pods. Lesions on lentil are lighter brown with a dark brown halo (Figure 9C). Lesions on pea tend to be more restricted, and the target pattern tends to be less obvious (Figure 9D). Under moist conditions, pinprick-sized, black pycnidia emerge in the center of the lesions. Lesions on plant tissue coalesce and cause defoliation, stem breakage, and lodging.
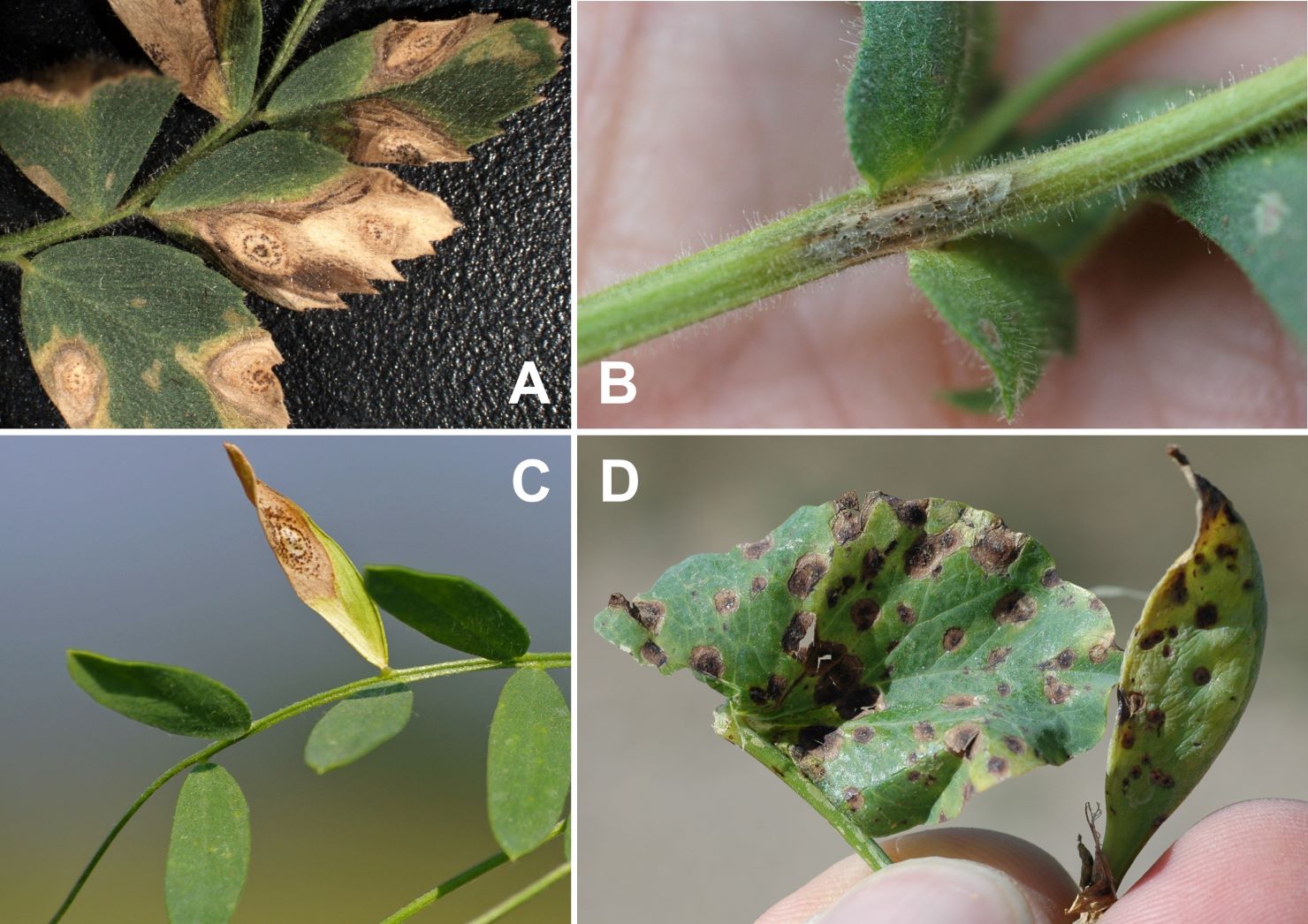
Figure 9. (A, B) Ascochyta blight on chickpea causes characteristic lesions with concentric rings on all above-ground plant parts. Ascochyta lesions on (C) lentil and (D) pea are less conspicuous and may be confused with bacterial blight or different fungal diseases.
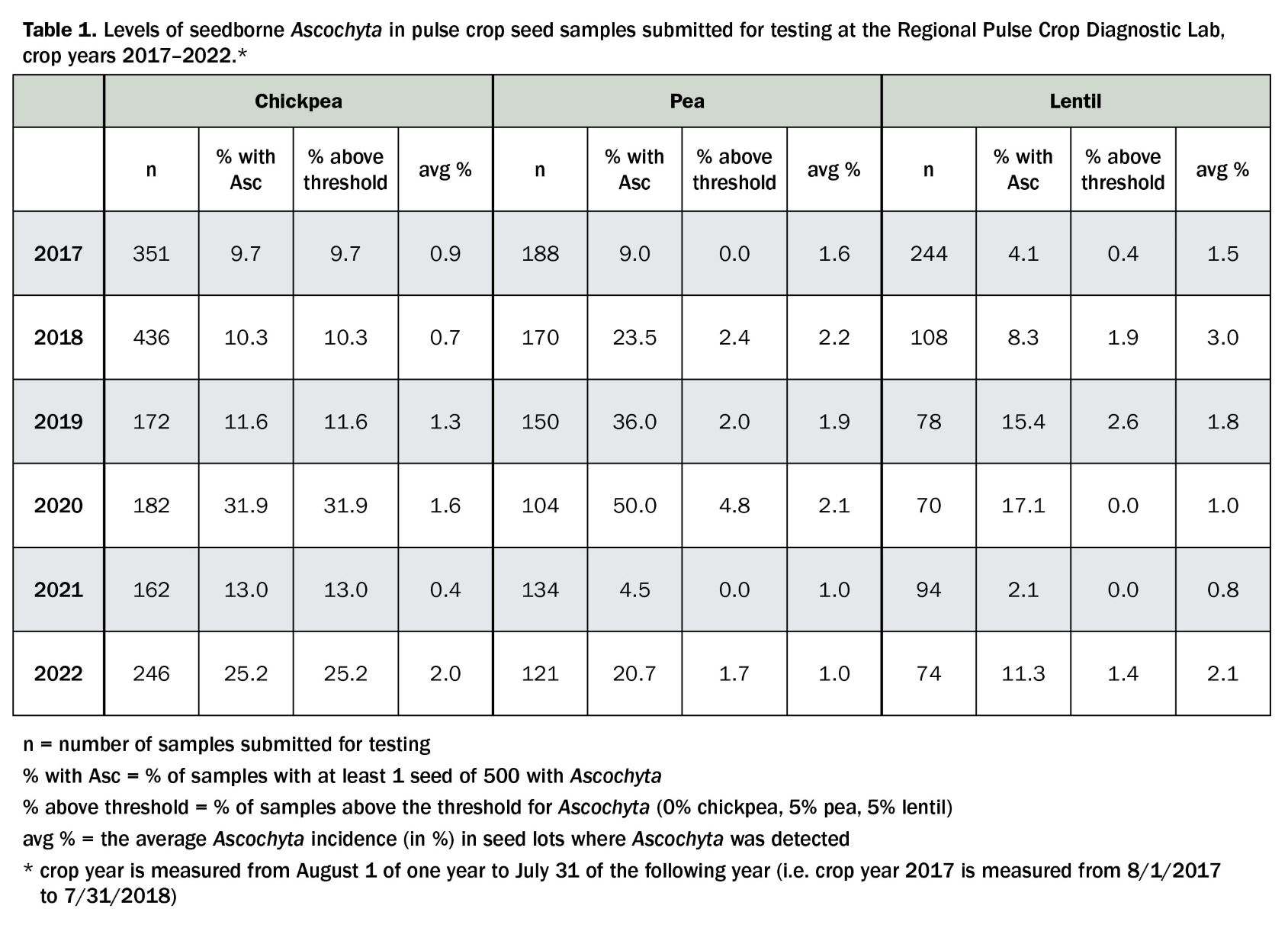
Management of Ascochyta blight begins at planting. The disease is residue-borne and rotation intervals of three to four years between the same legume crop are recommended to allow for pathogen levels on infested stubble to decline. Planting moderately resistant or tolerant cultivars delays disease development and spread and protects the crop from severe yield losses. The pathogen can be seedborne at high levels (Table 1). Planting pathogen-free seed can prevent the introduction of the disease into new fields. Montana and North Dakota seed labs will test for Ascochyta infestation of seed lots (see "Testing for seedborne pathogens of pulse crops"). A zero percent tolerance for Ascochyta on chickpea seed is recommended. For pea and lentil seeds, an Ascochyta incidence of less than 5% is advised. Several seed treatment fungicides can suppress the seedborne pathogen and prevent early infection of seedlings. Ascochyta spores can also be windborne and blow in from nearby fields with infested stubble. Within a field, the pathogen spreads via rain splash.
Environmental conditions favoring disease development include cool temperatures between 59°F to 77°F and high humidity. However, the disease can still develop under warmer conditions with adequate rainfall. Many foliar fungicides are effective against Ascochyta blight. The decision to apply a foliar fungicide will depend on the crop and variety, the timing of infection, and the incidence and severity of the disease. Given the rapid spread of this pathogen, fungicides will be most effective if applied as soon as disease is detected. Strobilurin-resistant strains of the Ascochyta pathogen in pea and chickpea have been detected in Montana and North Dakota. In North Dakota, resistant populations went from less than 10% of detections to almost 100% in a single year (2005-2006) due to the overuse of strobilurin (QoI) fungicides (Wise, 2009). The use of strobilurin fungicides for the management of Ascochyta blight is therefore not recommended. Alternative fungicide options include chlorothalonil as a protectant early in the season (before canopy closure). Triazole and SDHI fungicides can be applied at bloom initiation or when disease symptoms are first observed. Additional applications may be necessary depending on weather conditions. Mixing fungicide modes of action (MOA) and rotating between MOAs in subsequent applications is important to prevent the development of fungicide resistance.
Stemphylium blight (chickpea, lentil), caused by Stemphylium botryosum, is an emerging disease of importance on lentil in Canada, Montana, and North Dakota. It has also been observed on chickpea in Montana and North Dakota. Stemphylium causes defoliation of lentil and chickpea plants (Figure 10). Lesions often start at the edge of the leaflet, fuse with adjacent lesions, enlarge, and eventually cause leaflet drop. The lesions are often oval or irregular in shape, sunken, dark brown with lighter centers, and have a yellow halo. Older spots on chickpea may develop concentric rings resembling a target and may be confused with Ascochyta blight. In lentil, lesions tend to be more beige-colored, and dead leaves develop a characteristic rolled and twisted appearance (Figure 10). Effects on yield have been inconsistent. The pathogen survives on infected plant debris, seeds, and in soil. It prefers warm temperatures of 77°F to 86°F and moist conditions (85% relative humidity) with 6 to 12 hours of leaf wetness required for disease development. Lentil plants become more susceptible to Stemphylium as they mature, and the disease tends to develop following late-season rain events between late bloom and pod-fill. Infected seed has significantly lower germination rates, however, the significance of seedborne inoculum on disease development is unknown (Sharma & Joshi, 2021). The efficacy of fungicides for the control of this disease is largely unknown.

Figure 10. (Left) Stemphylium blight on lentil begins as tan lesions on the leaflet edges that expand to kill the leaflets, which take a characteristic twisted shape. (Right) Leaflets eventually drop, leaving the plants defoliated.
Septoria blight of pea, caused by Septoria pisi, occurs in Montana and is easily confused with Ascochyta blight due to the formation of small black spots (pycnidia) in lesions. The lesions can be yellowed, sunken, and irregular in shape (Figure 11). Unlike Ascochyta blight, pycnidia caused by Septoria blight are not arranged in concentric circles, and not all lesions are circular in shape. The disease is considered of minor importance.


Figure 11: Septoria lesions are irregular in (left) shape and (right) sunken. In contrast to Ascochyta blight lesions, pycnidia do not occur in concentric rings but are scattered in lesions.
Powdery mildew (Erysiphe pisi, E. trifolii, E. baeumleri, and Leveillula taurica) occurs sporadically in Montana and North Dakota and is generally late enough in the season that it is not economical to control. Early infections with powdery mildew can cause significant yield loss. The pathogen resides on infected plant stubble, leguminous weeds, or volunteer plants from previous crops. Infected plants are covered with a white powdery mass of spores that can be rubbed off the leaf during early disease development (Figure 12). As the disease progresses, the infected plant tissue turns gray and blue, and dark brown to black fungal structures emerge in the white mycelium. The disease develops at moderate to warm temperatures of 70°F to 85°F and is favored by overnight dew, but not rain. It can cause the crop to mature unevenly and bring about problems with the harvest. Desiccants will not be able to penetrate the mat of fungus and are thus not recommended. Fungicides are available for disease management early in disease development; however, variety resistance is the most economical means of control when available.
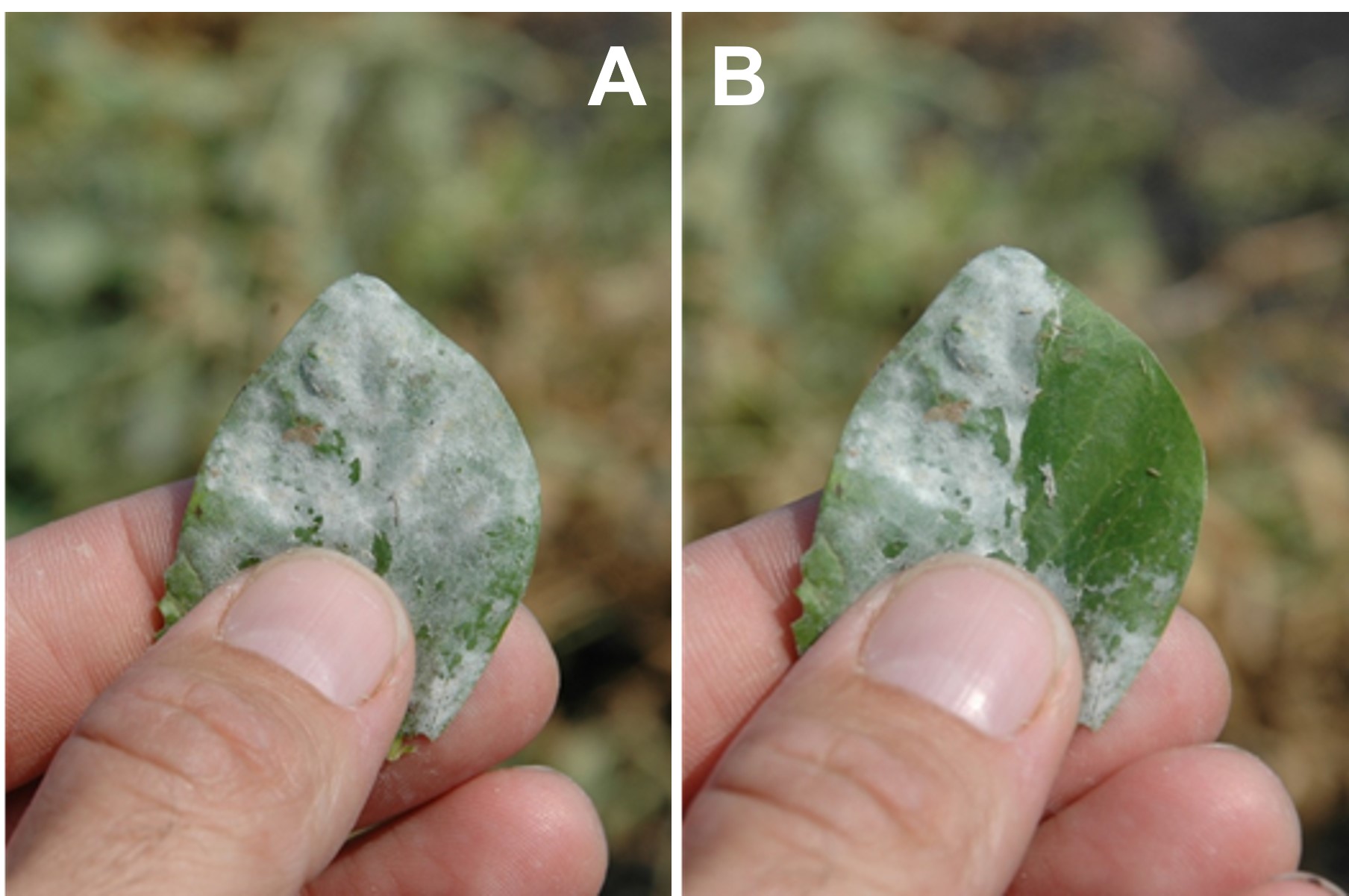
Figure 12: (A) Powdery mildew forms a white mat of fungal hyphae on the leaf surface and (B) can be rubbed off. Fungicides and desiccants cannot penetrate this thick layer of fungus.
Gray mold is uncommon in Montana and North Dakota but can be devastating when flower and pod infections occur. The disease is caused by Botrytis cinerea and associated species in chickpea and lentil. The pathogen attacks all aerial parts of the plant but prefers blossoms and pods. Symptoms initially start as water-soaked lesions, which progress to gray-brown lesions that are often covered with gray fuzz consisting of fungal hyphae and spores (Figure 13). Gray mold is favored by temperatures of 68°F to 75°F and high relative humidity (≥95%). Flowers commonly drop from the plant, which causes significant yield losses. The pathogen can also cause seedling soft rot in chickpea, which is the result of seedborne inoculum.
B. cinerea has a very wide host range of over 200 plant species including crops and weeds. The pathogen enters a field via infected seed, infested soil, and plant debris. It survives on plant debris and in the soil. Botrytis also survives on infected seed for up to five years. Seedborne transmission is an important source of the pathogen and seed should be tested. Seed treatment fungicides can effectively reduce seedborne inoculum. Resistant varieties, compact and erect growing cultivars, wide row spacing, reducing irrigation, intercropping with non-hosts such as wheat, crop rotation, and deep plowing help manage the disease.
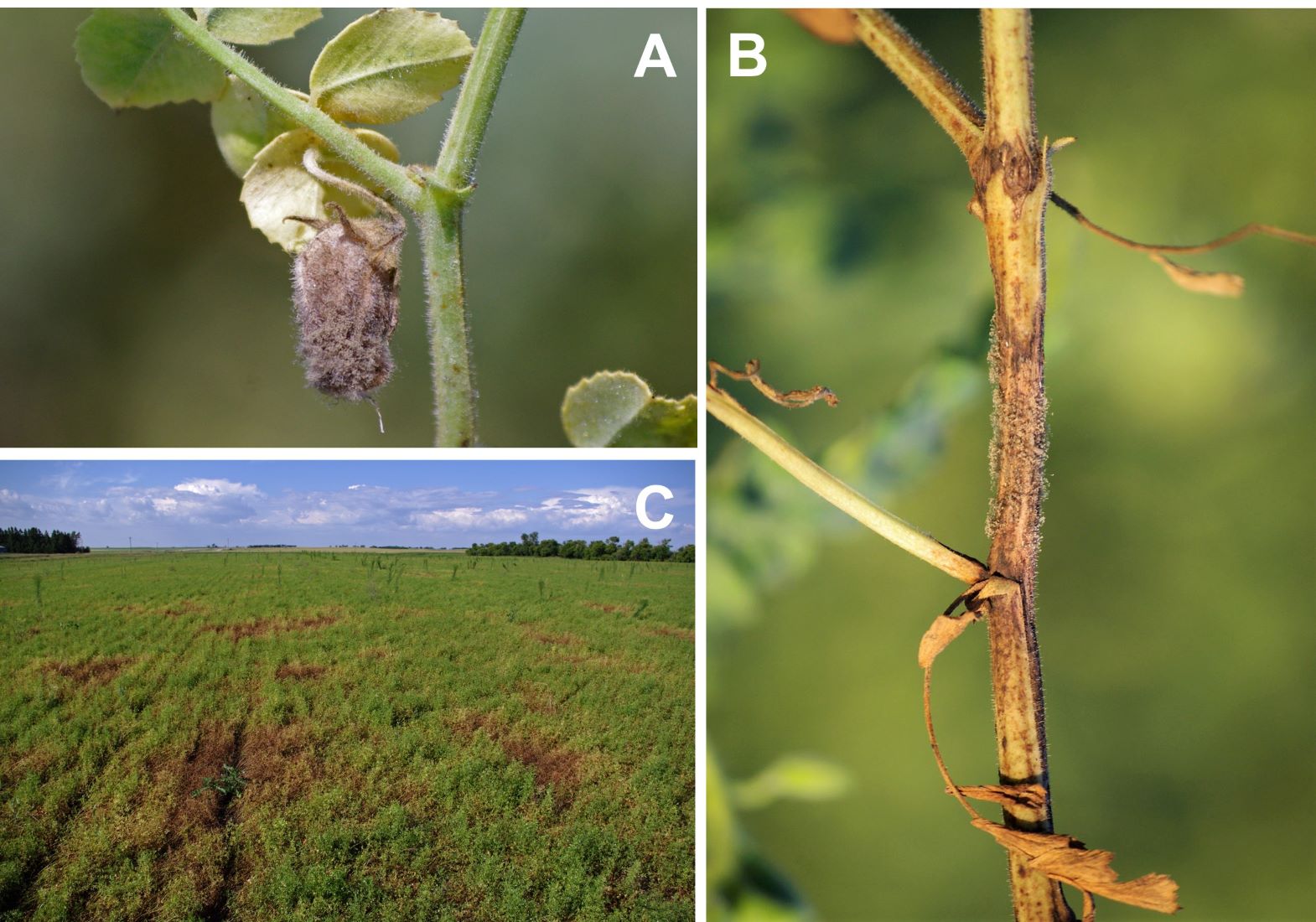
Figure 13: Botrytis gray mold on chickpea (A) pods, (B) stems, and (C) field symptoms on lentil.
Sclerotinia white mold and stem rot, caused by Sclerotinia sclerotiorum, S. trifoliorum, and S. minor, has a very broad host range (beans, mustards, potatoes, sunflowers, etc.) and infects many broadleaf crops and weeds. It often enters fields through contaminated seed lots or equipment. Spores can also spread short distances via wind from ditches or neighboring fields. The pathogen survives as sclerotia (Figure 14). Flowers become infected by ascospores, when temperatures are mild (70°F) and humidity is high, such as at canopy closure. The fungus can also grow directly from sclerotia into a plant through the root, stem, or other plant parts, especially if the plant has soil contact caused by lodging. The fungus will kill the host tissue and fill the stem with white hyphae and sclerotia, which then survive in the soil for many years. Infected plants appear wilted and are visible from above the canopy.
Sclerotia can be buried with tillage, but repeated tillage will bring them back to the soil surface. Management practices that reduce humidity in the canopy and prevent pod and stem tissue from coming in direct contact with the soil can reduce white mold. Triazole (Group 3) and SDHI (Group 7) fungicides are registered for the management of white mold. The timing of application and coverage is critical. Most fungicides should be applied at the flowering stage. Check the label restrictions before application. Crop rotation to non-host crops, such as small grains, reduces inoculum buildup and should be combined with good broadleaf weed control. Partial resistance may be available in dry pea and lentil cultivars. No resistance has been observed among chickpea cultivars.

Figure 14: (A) The white mold fungus produces sclerotia in and on the plant stem. (B) White mold growth can be visible as thick white mycelium on lower stems, (C) often near the soil line.
Management and control of fungal pathogens
Variety selection is an important disease control strategy, especially in areas that grow large acreages of pulse crops. Check with a retailer or local Extension agent for the best-adapted variety in the area. Some variety reactions to disease can be found in the Saskatchewan Ministry of Agriculture’s ‘Varieties of Grain Crops’ report (see “Helpful websites and contacts” section at the end of this publication).
Fungicide use recommendations are available from several sources. University sources include the MSU Extension Plant Pathology website, fact sheets on the High Plains IPM guide, MSU AgAlerts, presentations, and local Extension agents. The local fungicide salesperson or agronomist also has information. Information can also be found in the ‘NDSU Field Crop Plant Disease Management Guide’ and the Carrington Research Extension Center website. Many of the chemicals registered in North Dakota are registered in Montana, but always check the label. Section 18 and 24c (local) registrations are available on the Montana Department of Agriculture website. Links to the above-mentioned resources can be found in the “Helpful Websites and Contacts” section of this publication.
Viral diseases

Figure 15. Symptoms caused by virus infection are subtle and variable. They include, for example, (A) yellowing in mosaic-like patterns and (B) stunted growth.
Several viruses can infect pulse crops. The symptoms of infection can be subtle and easily confused with other disorders including nutrient deficiency or herbicide damage (Figure 15). Viral diseases of pulse crops are commonly transmitted by aphids and are much more common in the Pacific Northwest. Montana has been relatively free of virus issues thus far, which is an advantage of growing pulse crops here. However, in a year when aphid populations are high, we may see widespread virus diseases.
A common vector for many viral diseases of pulse crops is the pea aphid (Acyrthosiphon pisum). This aphid attacks pea, lentil, chickpea, alfalfa, clover, and some leguminous weeds. It can cause direct damage by feeding on plants, but it also vectors several important viruses. Alfalfa is a very good host of the pea aphid, so exercise caution when planting pulse crops near alfalfa. The University of Idaho has a legume virus project website with information on pea aphid thresholds, management, and an ‘aphid tracker’ tool that is updated throughout the season showing where aphids have been found and what viruses they carry: www.legumevirusproject.org/.
Viruses that have been found with some regularity in peas in North Dakota include Pea enation mosaic virus (PEMV), Pea seedborne mosaic virus (PSbMV), and Bean leafroll virus (BLRV). Other viruses which have been identified in legume crops include Alfalfa mosaic virus, Bean yellow mosaic virus, Cucumber mosaic virus, and Pea streak virus (not addressed in this publication).
Bean leafroll virus (BLRV, also known as pea leafroll virus) infects pea, chickpea, lentil, alfalfa, and other legume crops. It causes stunting, leaf yellowing and distortions, and leaf rolling in peas. Small brown lesions are often observed on infected leaves. The pea aphid (Acyrthosiphon pisum) is the principal vector of BLRV, although many aphid species can vector it. Aphids must feed for an extended time to acquire and transmit BLRV to new plants, so applying insecticides to control the aphid vector can be an effective strategy to manage the disease. Neonicotinoid seed treatments can limit primary infection by aphid vectors. BLRV was detected in Montana in chickpea, pea, and lentil in 2018.
Pea enation mosaic virus (PEMV) is one of the most important and destructive viruses of lentils worldwide and has been found in Montana. It also infects chickpea, pea, and other legumes. Symptoms include yellow to white mosaic patterns on leaves, stunting of the plant, twisted and malformed leaves, misshapen and poorly filled pods, and outgrowths (bumps) on the pod surface and the underside of leaves (Figure 16). Symptoms may be confused with growth regulator herbicide damage. Paying attention to patterns of symptom expression in the field can help discern the cause – random or unpredictable patterns are more likely due to disease, and systematic patterns are more often associated with abiotic causes, such as herbicide injury. The pea aphid and the green peach aphid (Myzus persicae) are the most important vectors of PEMV, but many other aphid species can transmit this virus also. Generally, aphid flights from other pulse-growing areas or neighboring fields carry the virus, and aphids may not be seen until the crop has been infected. Insecticides may be effective in reducing the secondary spread of PEMV.
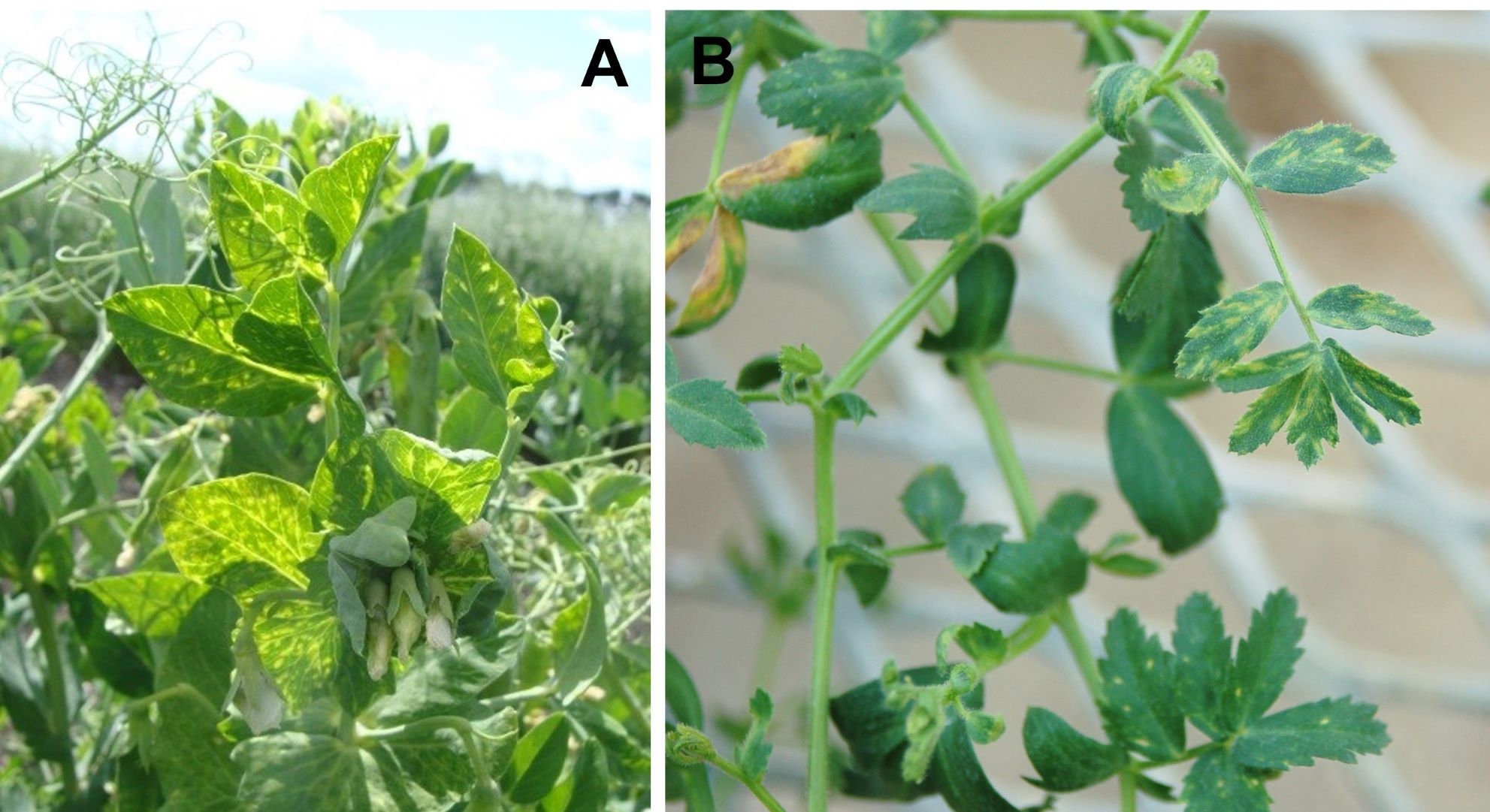
Figure 16: Symptoms of Pea enation mosaic virus (A)in field pea and (B) chickpea.
Pea seedborne mosaic virus (PSbMV) infects pea, lentil, and chickpea, and other legume hosts. PSbMV generally does not cause economic losses in chickpea. Symptoms associated with PSbMV include plant malformation (including pods) and stunted growth, terminal rosetting, leaf rolling, and delayed plant maturity. Leaflets may show indistinct mosaic patterns, reddening, or necrotic lesions. The seed coat of infected seeds can be cracked, split, or banded, but the virus can also be present in healthy-appearing seeds. Seed transmission is the most common mechanism by which the virus spreads in pea and lentil, while seed transmission is less than 1% in chickpea. Aphids facilitate the secondary spread of PSbMV. If aphid populations are high and not managed, PSbMV can spread from a few infected seedlings to large portions of a field during the growing season. The pea aphid, green peach aphid, and cotton aphid are the most common aphid vectors of this virus. The potato aphid can be of importance where potatoes are grown nearby. Planting virus-free seed is the most important strategy to prevent PSbMV outbreaks in a crop. Insecticides can reduce the secondary spread by reducing aphid vector populations but provide only partial control. Insecticide applications are most successful when they are well-timed to prevent economic aphid populations from being established, which requires regular scouting.
Glossary
Fungicide resistance: when a fungal plant pathogen is no longer controlled by a fungicide
Hyphae: the vegetative body of most fungi; long, thread-like filaments
Incidence (disease): ratio or percent of plants that have disease
Inoculum: a substance used to introduce a pathogen into a living organism
Oospores: thick-walled resting spores, characteristic for water molds (Oomycetes)
Pathogen: a living organism that causes plant disease
Polycyclic disease: two or more cycles of spore production and infection throughout the growing season
Pycnidia: fungal, spore-producing structures
Quantitative resistance: partial host resistance that can be combined with numerous resistance genes
Sclerotia: a hardened mass of fungal hyphae covered by an environmentally resistant black rind; microsclerotia are smaller sclerotia
Severity (disease): ratio or percent of plant tissue area with symptoms
Vascular tissue: the vessel-containing tissue that conducts water and nutrients throughout the plant
Vector: an organism that transmits a pathogen from one plant to another
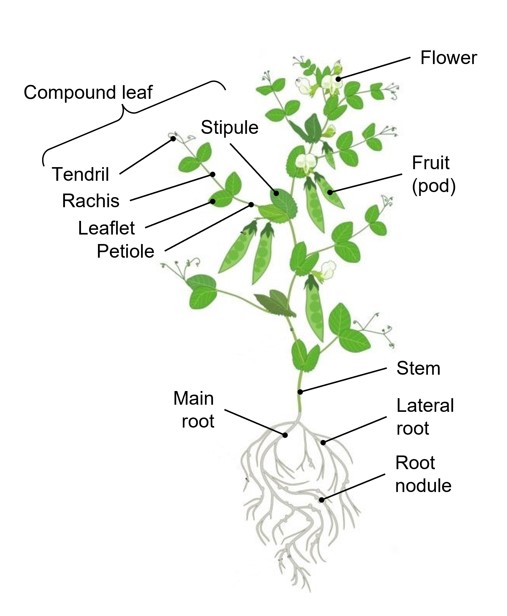 Figure 17: Morphology of a pea plant with main parts labeled
Figure 17: Morphology of a pea plant with main parts labeled
For Further Information
Agindotan, B., Fenoglio, J., Mahathar, M., McPhee, K., & Burrows, M. (2019). First Report of Bean Leafroll Virus in Chickpea, Lentil, and Dry Pea in Montana. Plant Disease, 103(5), 1050-1050.
Chen, W., Sharma, H., and Muehlbauer, F., eds. 2011. Compendium of chickpea and lentil diseases and pests. APS Press, St. Paul.
Gossen, B. D., Conner, R. L., Chang, K. F., Pasche, J. S., McLaren, D. L., Henriquez, M. A., Chatterton, S., and Hwang, S. F. (2016). Identifying and managing root rot of pulses on the northern great plains. Plant Disease, 100(10), 1965-1978.
Kraft, J. M., and Pfleger, F. L. 2001. Compendium of pea diseases and pests, 2nd ed. APS Press. St. Paul.
Ligoxigakis, E.K., Vakalounakis, D.J., and Thanassoulopoulos, C.C. 2002. Host range of Verticillium dahlia in cultivated species in Crete. Phytoparasitica. 30: 141-146.
Mansfield, P.J., Wilson, D.W., Heath, M.C., and Saunders, P.J. 1997. Development of pea bacterial blight caused by Pseudomonas syringae pv. pisi in winter and spring cultivars of combining peas (Pisum sativum) with different sowing dates. Ann. Appl. Biol. 131: 245-258.
Porter, L.D., Gunderson, B., and Inglis, D.A. 2009. Efficacy of phosphorous acid in managing Aphanomyces root rot in processing peas. Phytopathology. S104.
Sharma, S. and Joshi, L.P. 2021. Current insights on stemphylium blight of lentil with its management strategies. Sarhad Journal of Agriculture 37: 247-261.
Thakur, R.P., Girish, A.G., Rao, V.P., S. Asaad, and A. Moukahal. 2010. Fungi-chickpea. Crop Genebank Knowledge Base. Accessed 1 Sept 2010.
Wise, K. A., Bradley, C. A., Pasche, J. S., and Gudmestad, N. C. (2009). Resistance to QoI fungicides in Ascochyta rabiei from chickpea in the Northern Great Plains. Plant disease, 93(5), 528-536.
cropgenebank.sgrp.cgiar.org/index.php?option=com_content&view=article&id=457&Itemid=639
Helpful websites and contacts
Pulse Crop Information
North Central IPM Center Pulse Crop working group
Northern Pulse Growers Association
1710 Burnt Boat Drive
Bismarck, ND 58503
(701) 222-0128
Fax: (701) 222-6340
USA Dry Pea & Lentil Council
2780 W. Pullman Road
Moscow, ID 83843
(208) 882-3023
Fax: (208) 882-6406
Saskatchewan Ministry of Agriculture’s ‘Varieties of Grain Crops’
Fungicide Use Information
Montana Department of Agriculture Pesticide Product Registration
NDSU Field Crop Plant Disease Management Guide
NDSU Carrington Research Extension Center, Plant Pathology
Pulse breeding programs
Kevin McPhee, Pulse Crop Breeding
Montana State University
Nonoy Bandillo, Pulse Crop Breeding
North Dakota State University
Rebecca McGee, Grain Legume Genetics Physiology Research,
USDA-ARS Pullman, WA
Testing for seedborne pathogens of pulse crops
Contact each lab or vendor to get details on services, prices, sampling, and shipping methods.
Montana Regional Pulse Crops Diagnostic Laboratory
Department of Plant Sciences/Plant Pathology
P.O. Box 173145
Bozeman, MT 59717-3145
(406) 994-7738
https://plantsciences.montana.edu/pulsecropdiagnosticlab/
North Dakota State Seed Department
P.O. Box 5257
Fargo, ND 58105-5257
(701) 231-5400
SGS Seed Services North America, Inc
Laura Carlson, Sarah Dammen
1405 32nd Avenue
Brookings, SD 57006
(605) 692-7611
Discovery Seed Labs Ltd.
450 Melville Street
Saskatoon, Saskatchewan
S7J 4M2
(306) 249-4484
20/20 Seed Labs Inc.
507 - 11 Avenue
Nisku, AB T9E 7N5
+1 (877) 420-2099
Photo credits
Cover: Kevin McPhee, North Dakota State University
Figure 5: Carmen Murphy, Montana State University
Figure 7, 10, 13, 14B: Michael Wunsch, NDSU-Carrington
Figures 9B and 9D, 12: Sam Markell, NDSU
Figure 14A: Weidong Chen, USDA-ARS, Washington State University
Figure 16A: Kevin McPhee, MSU
Figure 16B: Lyndon Porter, USDA-ARS, Washington State University
Figure 17: albertapulse.com/growing-peas/ (edited)
All others by Mary Burrows, Montana State University
